PHARMACEUTICAL-GRADE COLLAPSIBLE ALUMINUM TUBES
Collapsible aluminum tubes are ideal packaging containers for medical products such as medicinal ointments, creams, gels, and lotions. Pharmaceutical-grade tubes are required to be non-toxic and hygienic while offering the highest possible degree of protection against contamination.
THE ENVIRONMENTAL SETTING
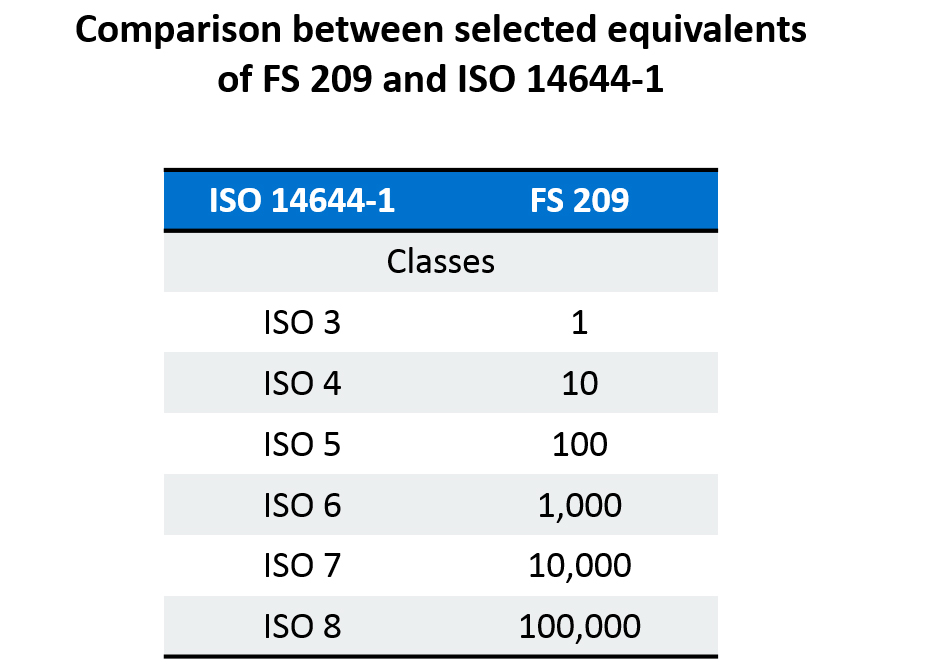
Xinrontube owns and operates a factory comprising eight (8) - ISO 8/ISO class cleanrooms, which fully conform with the ISO classification system ISO 14644-1 adopted by the primary authorities in the U.S. and Canada. Cleanroom classifications reflect the level of cleanliness of the air found inside them.
Here left is the new and the old editions for comparison purposes:The ISO standard 14644-1 (ISO 5, 6, 7, 8) replaced the Federal Standard 209 classification (Class 100; Class 10,000; Class 100,000) in 1999 (and revised in 2015), but many companies continue to use the traditional Class 100; Class 10,000; Class 100,000.
A series of crucial environmental parameters are monitored continuously in the cleanroom, including airborne particles, settling microbes, temperature, relative humidity, air pressure difference, and rate of air change. The surveillance of these environmental aspects remains constant throughout the entire production process.
Reference:
GB 50457-2019 Standard for design of pharmaceutical industry clean room
YBB 00412004-2015 Test methods for clean room to produce pharmaceutical packaging materials
THE PRODUCT SPECIFICATIONS
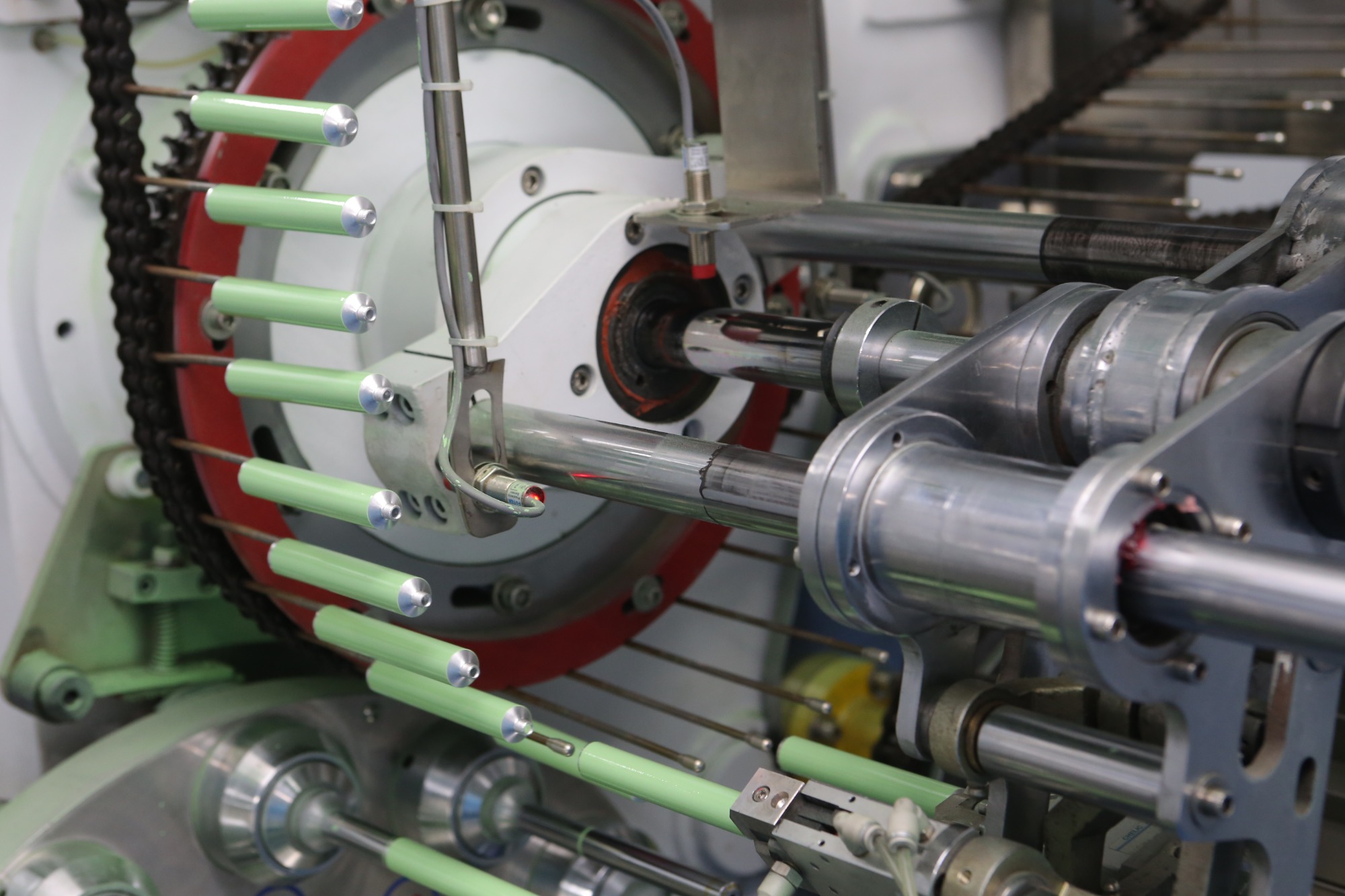
Xinrontube manufactures aluminum tubes used to package medical ointments for topical treatments and an array of other healthcare products. The aluminum ointment tubes sizes offered are 13.5 mm, 16 mm, 19 mm and 22 mm in diameter, with an applicable capacity of 5ml, 10ml, 15ml, and 20ml respectively. Xinrontube operates three automatic production lines. Annual production capacity can range as high as 100 million pieces. Because pharmaceutical packaging material is sensitive to environmental contamination, the production process is conducted under the strictest protocol possible.
DIMENSIONS
Xinrontube has obtained the production license for pharmaceutical packaging material from the China Food and Drug Administration (CFDA). The certification covers every physical aspect of the pharmaceutical- grade aluminum squeeze tube such as tube appearance, flexibility, chemical stability of internal lacquer, tube integrity, tube collapsibility, and microbial limits. Production standards for pharmaceutical grade collapsible aluminum tubes for ointments varies according to the primary location of the initial client as follows:
** For China, Pharmaceutical tubes made by Xinrontube comply with YBB00162002-2015.
** For EU, Xinrontube products follow European Standards approved by CEN.(Flexible aluminum tubes standards lists are available upon request)
** For U.S. clients, Xinrontube has filed for Type III DMFs with the FDA.

THE BENEFITS
GXPPC Ltd., provides a one-stop service to clients from around the world, and specializes in customized shapes and designs. Xinrontube aluminum collapsible pharmaceutical-grade tubes are produced in a totally contaminate-free environment and are packed carefully in corrugated cartons, either with or without partitions depending on the client's needs.
THE PRODUCT QUALITY-CONTROL
Product quality excellence is the core mission at Xinrontube and accomplished using a three-tier system:
1. The use of a KPI key indicator management assessment system provided to all operational personnel, which precisely identifies and prioritizes the quantity and quality for each day's production.
2.The use of and strict adherence to a quality management system that synchronizes both the international standards and the customers prevailing requirements as described within these noted publications: Primary packaging materials for medicinal products — Particular requirements for the application of ISO 9001: 2015. Reference to good manufacturing practice (GMP) Quality Management System - ISO 9001 International Standards.
3. The use of a CCD computer visual inspection system which employs a high-efficiency CCD photo-electric detection system to monitor appearance quality and product integrity stringently. The utilization of such a system has obvious benefits over manual inspection by eliminating aspects such as visual/physical human fatigue and is ideal for providing maximum control of product quality.